We value your privacy.
We use cookies to enhance your browsing experience, serve personalized ads or content, and analyze our traffic. By clicking "Accept All", you consent to our use of cookies.


Explore our breast reconstruction and augmentation solutions designed with high-end technology and research-based knowledge focused on improving patient safety and aesthetic outcomes.
Our Commitment to Surgeons
Both your well-being and success as a plastic surgeon matter to us; we are dedicated to supporting you.
Our Mission
We strive to create safe solutions based on science and user-centric design through collaboration, integrity and respect.


Our Vision
We aspire to transform our industry by focusing on women’s health and creating value for all stakeholders.

At Motiva®
We stand by the independence of today’s women, and the evolution of beauty
through cutting-edge technology and international quality standards.
Discover The Motiva® Standard

Women’s Choice
With over 3,000,000 implants in the market, Motiva® continues to be the top choice in comfort and aesthetics.


Committed to You
Our commitment to women’s health and well-being is supporting each and every woman through the evolution of her life.
Motiva® Implants Overview
Explore all the different options, programs, and technologies of our next-generation implants: created for personalized results you can trust.


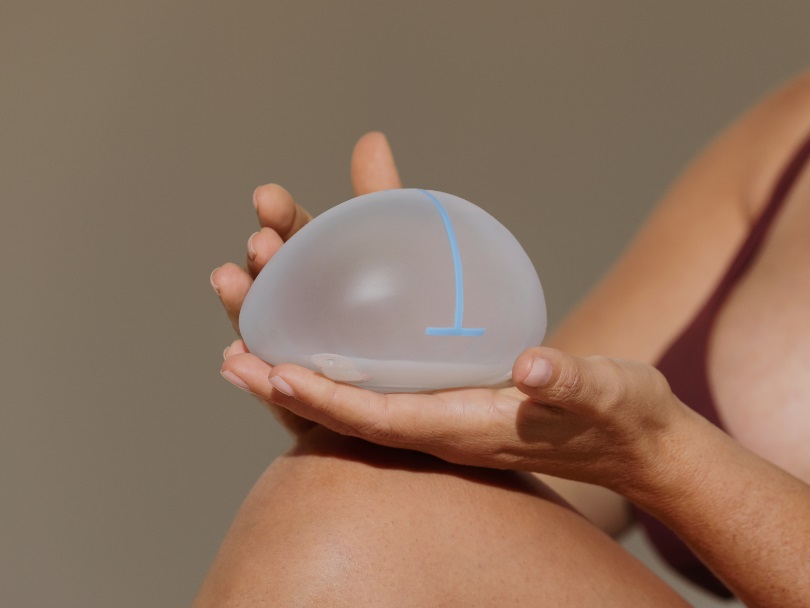
Ergonomics – the science behind designing products to be as user friendly as possible – is a paramount part of our patient-centric mandate, which is why we challenged ourselves to come up with an implant that mimics the dynamics of natural breast tissue.
Ergonomix® comes in standard (round) and oval bases to suit a variety of thorax shapes. Our oval-based version is shaped to better suit women with longer or wider chest profiles and possesses radiopaque lines that distinguish between its vertical and horizontal positions.



Our Social Commitment to Women
Sustainability is one of our biggest pillars and it begins with our commitment to Women’s Health it is practised by our daily actions to promote well-being, empowerment, and the protection of our planet.
All our processes, products, employees, and business partners are aligned with our commitments to women’s health, sustainable development, and long-term value and well-being for our stakeholders.
We embraced different international frameworks, like the United Nations Sustainable Development Goals (SDGs) and the Women’s Empowerment Principles to promote gender equality and women’s empowerment in our industry and support breast health programs and quality breast reconstruction initiatives.
All these reflect our purpose as a global corporate citizen: “Putting Women First: in health and wellbeing, science and technology, and our communities.”


Breast Cancer and Breast Reconstruction Initiatives
We know every breast cancer journey is different, and we stand by each woman to tell her that hers is important to us – whether it is prevention, detection, battle, or survival. We are their partners.
Driving women’s empowerment
In 2018, we created Beauty Boss, a global networking initiative to empower women in plastic surgery by sharing strengths & discussing challenges. It is a session for women to talk and share about their careers, family, professional development, and self-improvement.


Motiva® & Chess
To have a positive impact on the world and feel truly empowered, we all need to live our lives to the fullest. This is why we encourage all women to seek the best version of themselves. Chess showcases intellectual skills, formidable strategy, and mental and physical strength – all at high speed. Our goal is to empower women to make smart, beautiful choices, just like a chess game!

We are carbon-neutral
In May 2019, our headquarters and manufacturing plants were certified as Carbon Neutral. We achieved this certification through a combination of efforts aimed to reduce our carbon footprints, such as the use of a more efficient lighting system for our facilities, solar panels and energy storage systems to power our operations, ice banks, and a special air conditioning system.
Sharing our Environmental Commitment
As part of our environmental goals, we shared our commitment to sustainable development and the environment with our employees. On September 6th, 2019, we organized an activity in which 22 volunteers from our headquarters joined efforts to clean a beach.

Aesthetic BreastRecon®
A new breast reconstruction platform designed to enhance women’s beauty and safety
Let’s keep innovation blooming!

We are confident that our commitment to women´s well-being and our innovative technologies around Aesthetic BreastRecon® can help women feel like themselves again, by bringing them solutions that enhance both beauty and safety.
Meet the Motiva Flora® Tissue Expander!
Motiva Flora® is a revolutionary MRI conditional tissue expander, meaning it is not contraindicated during MRI scanning.
Constructed with state-of-the-art technologies, this tissue expander provides women, undergoing two-stage breast reconstruction, with safety and stability during the tissue expansion process.
State-of-the-art Technology

Let’s keep innovation blooming
with Motiva Flora®!
MRI conditional technology
Conventional tissue expanders may have complications with their magnetic components during vital MRI scanning. As a result of these complications, conventional tissue expanders have been classified as unsafe for MRI scanning.
The Motiva Flora® Tissue Expander was purposely built with a nonmagnetic port in its detection system to offer women a safe solution to MRI scanning during the tissue expansion process.
The advanced technology of the RFID port holds MRI conditional labelling, thus proven to reduce drawbacks seen in conventional tissue expanders.
What makes us unique:
Hybrid ergonomic reconstruction

Implant-based reconstruction (IBR) is a more popular procedure for multiple reasons: reduced donor site mobility, reduced scarring, and shorter operating and recovery times in comparison to autologous reconstruction.
Hybrid ergonomic reconstruction is the concept of combining autologous tissue with Motiva® Implants, offering patients the option of a natural-looking result.
Hybrid ergonomic reconstruction offers several advantages over implant-based reconstruction alone:


Let’s keep innovation blooming with Aesthetic BreastRecon®
Motiva® Designed Surgeries
Motiva® combines innovative products and tools with the most advanced surgical approaches to create designed surgeries for custom patient outcomes.


MotivaHybrid®
MotivaHybrid®
MotivaHybrid® combines lower-volume Motiva® breast implants (associated with fewer risks and less tissue strain than larger ones) with high-quality fat grafts made using a fat grafting system.
This hybrid procedure is perfect for patients who require detailed corrections (such as an inner cleavage boost) and high precision, especially if they would like to benefit from additional slimming, correction, or enhancement in other areas of the body.


Consider mommy makeover surgical packages, for example. A typical package would include volume reduction in some areas (such as the tummy, hips, and thighs) and volume addition in others (such as the breasts, buttocks, or hollowed-out areas of the face).
Harvesting of fat and lipofilling of fat grafts during MotivaHybrid® is performed using our proprietary Bulb-Cannula Kit– a patented, single-use system that has been designed and tested to protect breast implant integrity during fat grafting procedures.
Custom Size and Shape for Predictable Results


Motiva MinimalScar®
Motiva MinimalScar®
Smaller scars, quicker procedure, faster recovery.
With its unique combination of tools and techniques, the Motiva MinimalScar® procedure enables surgeons to insert Motiva® Implants using incisions as small as 2.5 cm – around half the size of the current industry standard.
Motiva® Implants are uniquely flexible and durable enough to be inserted through smaller incisions, thanks to their rheological properties and surface technology relative to other traditional implants.


Minimal-Incision Breast Augmentation

We are in this together
Let us walk you through the breast cancer journey and help you understand your options.

The Motiva® Experience
Your beauty.
Your health.
Your standards.
We are setting the safety standards for your breast health journey.


Your safety and peace of mind are worth it
Registering your implants is one of the most important steps towards
an amazing, stress-free breast augmentation journey.
You are in control
Have a positive, empowering experience customizing your breast
health journey with your desired look and feel.


Thank you for being our partner in this evolution journey!
We have developed our partnership platform in order to continue supporting your growth.

Motiva® Implant Matrix
Use our Motiva® implant matrix to find over 600 product options for tailored results.

Motiva® Clinic App
A dynamic, digital environment to facilitate interaction with your patients.

MotivaEDGE®
Events
Be a part of our global platform dedicated to medical education with our global webinars and live events.

Let Patients Find You!
Our Center Locator is designed to connect patients with Motiva® partners.

Register Implant
Support
Need a hand? Click on the button below to find useful information about Motiva®.
This information is intended solely for the use of healthcare professionals. A healthcare professional must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Motiva® does not dispense medical advice and recommends that healthcare professionals be trained in the use of any particular product before using it in a procedure or surgery. A healthcare professional must always refer to the package insert, product label and/or instructions for use before using any Motiva® product. The information presented is intended to demonstrate particular products, as well as the breadth of Motiva® product offerings. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your local representative if you have questions about the availability of specific products in your area. Motiva® products that are CE-marked are marked according to the applicable EU Regulations and Directives.
Yes, Motiva Implants®* are FDA approved with less than 1% device related-complications¹.
Reference:
(1) Glicksman C, Wolfe A, McGuire P, The Study of the Safety and Effectiveness of Motiva SmoothSilk Silicone Gel-Filled Breast Implants in Patients Undergoing Primary and Revisional Breast Augmentation: Three-Year Clinical Data. Aesthet Surg J. 2024 sjae134, doi.org/10.1093/asj/sjae138
We manufacture Motiva Implants, but we don’t do any surgeries.
Surgeons from all over the world are using our implants. You can find our Center locator, where certified surgeons who use Motiva Implants are listed in your requested region down below.
We suggest that you make an appointment with one or more of them to discuss the desired result and Motiva options.
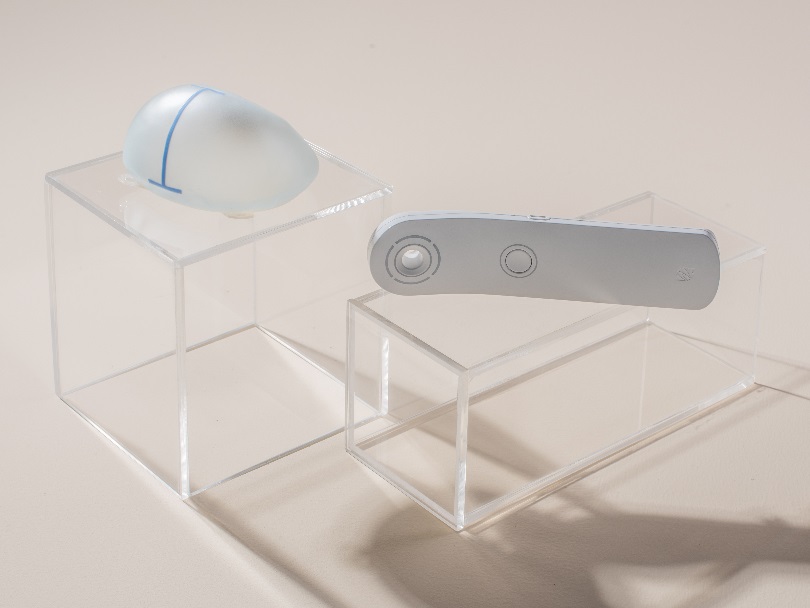
Qid® is the world’s first microtransponder for clinical use in a breast implant. It is the only transponder with a CE mark (which indicates compliance with the health, safety, and environmental protection standards for products sold in the European Economic Area), and the United States Food & Drug Administration (FDA) cleared it for use in 2004. Qid® is placed in the gel filling inside Motiva® Implants.
A handheld reader is used to externally scan the transponder, providing an electronic serial number (ESN) that can be used to retrieve important implant information such as manufacturing date, implant type, volume, and more.
Qid® is an optional feature in Motiva® Implants that is particularly useful to patients and surgeons to identify the implant type after the surgery, and to verify implant details during the event of a product recall.
Product information or warranty on cards can be lost or misplaced. With its unique ESN permanently located within the breast implant, Qid® provides the confidence of instant implant traceability and verification.
A radio frequency identification (RFID) microtransponder (Qid®) is a passive component (without a battery) placed in the gel that provides each implant device with a unique electronic serial number which is only accessible through the use of a handheld scanner specific to Motiva. The Electronic serial number does not contain patient-specific or identifiable information, only device-specific information such as the manufacturing date, serial number, lot number, implant volume, size and projection, model and surface type).
The American Academy of Pediatrics has stated that there is no reason why a woman with implants should refrain from nursing. However, breast implant surgery may interfere with how optimal breastfeeding may be, either by reducing or eliminating milk production.
Most women with breast implants who attempt nursing have successfully breastfed their babies. It is not known if there are increased risks for a woman with breast implants or if the children of women with breast implants are more likely to have health problems.
Please inform your surgeon in advance of your procedure if you wish to have the option to breastfeed afterwards, as a surgical approach with this consideration may reduce the chance of breastfeeding difficulties.
The microtransponder (Qid®) is MRI-safe. The device is “MRI conditional”, meaning that it has been demonstrated to pose no known hazards in a specified MRI environment with specified conditions of use.
Qid® may create an imaging void during MRI (known as an artifact effect) that can block visualization of a small area of minor breast tissue near it. In special cases, alternative imaging techniques such as ultrasound, tomosynthesis, digital compression mammography, subtraction contrast mammography, and/or scintimammography can be used to better visualize the region obstructed by Qid®.